Last Updated on June 30, 2020
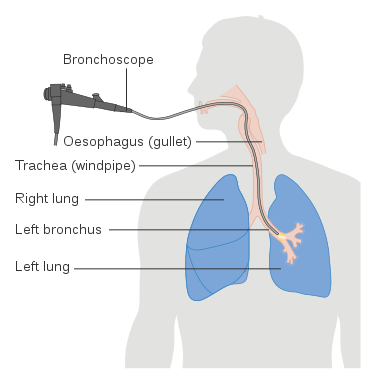
Bronchoalveolar lavage (BAL) is a procedure in which a bronchoscope is passed through the mouth or nose into the lungs to obtain cells and other components from bronchial and alveolar spaces for diagnostic purposes.
It is performed to diagnose various lung diseases including infections and malignancies.
Bronchoalveolar lavage is a diagnostic procedure similar to flexible bronchoscopy (in which a bronchoscope is wedged into a bronchus). In addition to the insertion of a bronchoscope, BAL involves pumping of sterile saline into the broncho-alveolar pathway which is then retrieved along with the fluid and cells to be analyzed.
Bronchoalveolar lavage is a minimally invasive, first-line examination of the lung parenchyma.
Bronchoalveolar lavage has an important diagnostic role in clinical pulmonary medicine. The fluid obtained through this procedure can be used for cytology examination, Gram staining, and culture and sensitivity. In addition, many other biomarkers can be analyzed from BAL fluid, thereby helping the clinician in making a diagnosis.
The right middle lobe (RML) and lingula of the lung are more easily accessible and are likely to allow a good return of lavage fluid with patients placed in a supine position. Traditionally, these sites have been used for performing BAL.
However, with the use of high-resolution computed tomography (HRCT), images of the lung can be obtained which show specific areas having a prominent change (especially areas with ground-glass attenuation). Lavaging these areas increases the chances of obtaining diagnostic material.
Bronchoalveolar lavage is distinguished from segmental or whole lung lavage (WLL) which is a therapeutic procedure usually employed in pulmonary alveolar proteinosis to wash out the proteinaceous material occluding the airspaces. It is an infrequently done procedure carried out only in a few tertiary care centers.
Bronchoalveolar lavage is different from bronchial lavage. In the latter, saline is instilled into the large airways or bronchial tubes and then aspirated for fluid analysis. This results in the sampling of larger airways. It is carried out to detect cancer or to identify infectious pathogens involving the larger airways. In contrast, during BAL, the bronchoscope is directed into a smaller airway to minimize sampling from the large airways. BAL may be focused on specific regions of the lung, and multiple lavages may be performed during the same procedure.
Indications of Bronchoalveolar Lavage
Pulmonary Infection
- Immunocompromised host with infiltrates
- Ventilator-associated pneumonia
- Non-resolving pneumonia
- Diffuse lung infiltrates (interstitial and/or alveolar)
Pulmonary Malignancy
- Lymphangitic carcinomatosis
- Bronchoalveolar carcinoma
- Other malignancies
Acute Respiratory Failure
Diffuse Infiltrative Lung Disease
- Alveolar hemorrhage
- Sarcoidosis
- Pulmonary alveolar proteinosis
- Eosinophilic pneumonia
- Drug toxicity
- Pulmonary Langerhans cell histiocytosis
- Hypersensitivity pneumonitis
- Idiopathic pulmonary fibrosis
Occupational Lung Disease
- Chronic beryllium disease
- Asbestosis
Post-transplant Monitoring of the Lung Allograft
Pediatric Lung Disease
- Infection
- Interstitial Lung Disease
- Aspiration
- Hemorrhage
- Cystic fibrosis
Procedure of Bronchoalveolar Lavage
Equipment Required
• Flexible bronchoscope
• Sterile collection trap
• Suction tubing
• Sterile saline
• Vacuum source
• Syringe
• Lidocaine 1-2%
Pre-Procedure Preparation
BAL is mostly done under conscious-sedation anesthesia as an outpatient procedure. However, it can be carried out even in critically ill patients.
The patient should be fasting overnight so as to reduce the risk of aspiration. Medical conditions such as COPD or other should be stabilized before the procedure is done.
To avoid contamination of the specimen, BAL should be performed prior to any other bronchoscopic procedure.
X-ray and HRCT images should be evaluated to determine the ideal site for carrying out lavage.
Written consent is obtained from the patient/ relative.
Patient Preparation
- Supine position.
- Apply all the apparatus
- Supplemental oxygen
- Monitoring equipment – ECG, pulse oximetry, blood pressure cuff, etc.
- Administer bronchodilators when there is a risk of spasm. Warm saline would induce lesser bronchospasm.
- Sedate the patient in order to reduce patient discomfort and minimize cough reflex
- Benzodiazepine – midazolam or other
- Opioid – Fentanyl or other
- Avoid topical anesthesia with an excessive amount of lidocaine as lidocaine may have bacteriostatic effects.
- Prepare aliquots of saline containing 20 to 60 mL of saline each, with six aliquots for a single lavage site.
Technique of Bronchoalveolar Lavage
- Insert the bronchoscope into the airways, through the nose or mouth.
- After inspection of the airways, the bronchoscope is advanced and wedged into a smaller airway (a fourth-division or fifth-division subsegment of one of the lobar bronchi).
- Avoid bronchial trauma especially in patients with suspected alveolar hemorrhage.
- Infuse the saline contained in one aliquot with a syringe into the distal airway through the suction channel of the bronchoscope. The flow of saline at the distal tip of the bronchoscope is observed.
- Aspirate back quickly by gentle suction (50-80mmHg)
- At higher negative suction pressures, distal airways may collapse leading to decreased fluid recovery. If it happens, reduce the suction pressure.
- Instructing the patient to inhale and exhale deeply may improve the return.
- Collect the lavage specimen in the collection trap.
- Repeat the infusion of saline and aspiration up to 6 times The total instilled volume should be about 100 to 250 ml.
- In the case of reduced flow, the re-orientation of the bronchoscope tip allows a better return of fluid.
- Keep the patient under observation for 1 hour, with continued monitoring.
Complications and Adverse Events
The majority of the patients show no major adverse effect or complication during or after the procedure.
Most of the reported adverse effects are transient. These include
- Cough
- Transient hypoxemia
- Dyspnea
- Transient fever
- Transient chills and myalgias
- Bronchospasm
- Transient decrease in baseline PaO2
- Transient fall of lung function
In patients with severely compromised respiratory status, the loss of lung function may necessitate the need for mechanical ventilation.
If a biopsy has been performed along with BAL, then a chest radiograph must be carried out after the procedure to rule out pneumothorax.
Read more about Types of Biopsies and Their Applications
Diagnostic Testing of Bronchoalveolar Fluid
The results of BAL analysis must always be interpreted in the context of clinical findings and imaging studies. It can provide a definite diagnosis in many lung diseases. Even if the findings are not diagnostic, BAL can provide sufficient information that helps to rule out suspected diagnoses and investigate some alternative diagnoses.
Collection and Handling of the Bronchoalveolar Fluid
The fluid should be collected in a clean and sterile container that does not promote adherence of cells to container surfaces (e.g., silicone-coated glass or polypropylene).
The sample should be transported to the laboratory and processed as soon as possible, preferably within one hour or maximum 2-3 hours.
If a delay is unavoidable, the fluid should be stored in nutrient-supplemented media for up to 12 to 24 hours. Beyond 24 hours, the specimen becomes unsuitable for analysis and must be discarded.
Labware used should also not promote cell adherence to container surfaces. Specimens with gross mucus can be strained through loose gauze, or small amounts of mucus can be dissolved with dithiothreitol, if necessary.
A small amount of specimen is then centrifuged at an appropriate speed, resuspended, and analyzed.
The remaining fluid is then refrigerated at 4-8◦C for up to 24 hours. Cells that were already suspended in a nutrient supplemented medium due to delayed transport can simply be refrigerated at 4-8◦C.
Gross Examination
Note quantity, color, turbidity, and appearance of fluid. In pulmonary alveolar proteinosis, the lavage fluid is opaque or translucent brownish or sandy colored. The sediment settles down at the bottom when the fluid is allowed to stand for some time.
In alveolar hemorrhage, the lavage becomes more hemorrhagic with each sequential aliquot.
Cell Counts and Differential
These are obtained via a hemocytometer or automated cell counters. The total number of red blood cells, white blood cells, their differential percentages, the percentage of epithelial cells, macrophages, etc are noted.
Alveolar macrophages
Macrophages from alveolar spaces constitute >80% of the cell population in a normal person.
Neutrophils
These constitute <3% of the total cell population. Increased counts are observed in active alveolitis, acute infections of broncho-alveolar pathways, aspiration pneumonia, idiopathic pulmonary fibrosis (IPF), acute respiratory distress syndrome (ARDS), connective tissue disorders, Wegener’s granulomatosis, pneumoconiosis, etc.
Lymphocytes
These constitute < 15% of the total cell population. In addition to total lymphocytes, BAL fluid can also be analyzed for the ratio of CD4+ and CD8+ T-cells. (using fluorescence-labeled monoclonal antibodies and flow cytometrical analysis).
Based on these calculations, the conditions showing lymphocytosis are classified into the following categories:
- Normal CD4/CD8 ratio – Tuberculosis, malignancies.
- Increased CD4/CD8 ratio – Active sarcoidosis, berylliosis, asbestosis, Crohn’s disease, connective tissue disorders.
- Decreased CD4/CD8 ratio – Hypersensitivity pneumonitis, silicosis, drug-induced lung disease, HIV infection, bronchiolitis obliterans organizing pneumonia (BOOP)
Eosinophils
These constitute < 1-2 % of the total cell population.
- Low to moderate eosinophilia (5-20%): Drug-induced lung disease (e.g. minocycline, nitrofurantoin, penicillin), infections (parasitic, mycobacterial, fungal), asthma, malignancies, other interstitial pneumonia occasionally (BOOP, IPF/UIP, ILD associated with connective tissue disorders).
- Moderate to marked eosinophilia (>20%): ABPA, Churg-Strauss syndrome, acute eosinophilic pneumonia, chronic eosinophilic pneumonia, idiopathic hypereosinophilic syndrome
Erythrocytes (RBCs)
Increased erythrocyte count is an early sign of alveolar hemorrhage (first several hours)
Cytology
A portion of BAL fluid is taken and centrifuged. Smears are prepared from the sediment obtained and suitably stained (Wright-Giemsa or May-Grunwald-Giemsa stain). The stained smears are then examined under the light microscope. The following features are noted.
Read more about Light Microscope: Parts, Usage, Handling, and Care
Epithelial Cells
Very few squamous epithelial cells and ciliated columnar epithelial cells are normally seen in BAL fluid specimens. The presence of a large number of squamous epithelial cells suggests that BAL fluid is contaminated with upper airway secretions, and the presence of large numbers of bronchial epithelial cells suggests that the BAL may not have adequately sampled distal airspaces.
Hemosiderin laden macrophages
The presence of more than 20% of these is highly suggestive of alveolar hemorrhage.
Phagocytosed erythrocytes
These are seen in cases of alveolar hemorrhage.
Cytomegalic cells
The presence of large cells (cytomegalovirus) or multinucleated cells ( herpes) is indicative of viral pneumonia.
Malignant cells
The smears are screened for the presence of any atypical appearing cell suggestive of malignancy.
• Bronchoalveolar carcinoma and other primary lung malignancies
• Lymphoma
• Extrapulmonary malignancies
Sulfur granules
These are seen in Actinomycetes infection
Langerhans cells
The presence of >5% of Langerhans cells is suggestive of Pulmonary langerhans cell histiocytosis.
Dust particle inclusions
Seen in cases of pneumoconioses, asbestos exposure.
Special Stains
Oil red O stain
For detecting neutral fat droplets that can be seen in fat embolism.
Fat and Lipid stain (e.g. Sudan III)
It is also a fat stain that is done in cases of Lipoid pneumonia ( pneumonia caused due to aspiration of lipids or oil).
Microbiology of BronchoAlveolar Lavage Fluid
Aerobic and anaerobic cultures
Aerobic and anaerobic cultures are done to look for bacterial growth
Special Stains
- Gram stain: Bacterial infection
- KOH preparation: Fungal infection
- Periodic acid-Schiff (PAS): Pulmonary alveolar proteinosis
- Ziehl-Neelson (ZN stain), Auramine-rhodamine, Auramine-O: Mycobacterial infection
- Modified acid-fast stain (Kinyoun stain): Nocardial infection
- Silver methenamine: Pneumocystis carinii pneumonia, fungal organisms
Polymerase chain reaction (PCR)
For Mycobacteria tuberculosis
Other investigations
Immunohistochemistry
A battery of immunohistochemical markers can be applied to BAL fluid. This is done to confirm and subtype the various malignancies detected during the cytological examination.
Electron microscopy
It is rarely indicated for diagnostic purposes but has a major role in research. Birbeck granules or “X” bodies (pentilaminar cytoplasmic inclusions) are indicative of Langerhans cells. Myelin like ultrastructure with lamellar bodies and myelin are seen in alveolar proteinosis.
Read more about Electron microscope – Types, Uses, and Disadvantages
Molecular techniques
Though not routinely performed, various molecular techniques (nucleic acid microarray analysis, proteome analysis, gene analysis, etc) can be carried out on the BAL sample for molecular characterization of lung diseases.
References
1. Wang K, Mehta A, Turner J, editors. Flexible Bronchoscopy. 2 ed. Ann Arbor, MI: Blackwell Science; 2004.
2. Prakash UBS, editor. Bronchoscopy. First ed. New York, NY: Raven Press, Ltd.; 1994.
3. British Thoracic Society Bronchoscopy Guidelines Committee aSoSoCCoBTS. British Thoracic Society guidelines on diagnostic flexible bronchoscopy. Thorax 2001;56 Suppl 1:i1-21.
4. Costabel U. Atlas of Bronchoalveolar Lavage. First ed. Philadelphia, PA: Chapman and Hall; 1998.
