Last Updated on October 29, 2023
The immune system is defined as a system of many biological structures and processes within an organism that protects against disease. The immune system must detect pathogens, from viruses to parasitic worms, and distinguish them from the organism’s own healthy tissue.
There are two broad categories of immunity involved in host defense – natural or innate and acquired. While natural immunity is more generalized, acquired immunity is more specific and adaptive. There are various levels of host defense that protect human body from invading attacks.
These are:
Physical barriers
- Natural (Innate) immunity: Skin, mucous membranes
- Acquired immunity: Mucosal immune system
Circulating factors
- Natural immunity: Complement, C- reactive protein
- Acquired immunity: Antibody
Cells
Natural immunity: Macrophages, Neutrophils, Natural killer cells
Acquired immunity: Lymphocytes
Cytokines
Natural immunity: Monokines (IL-1, TNF, IL-12, interferons)
Acquired immunity: Lymphokines (interleukins)
Active and Passive Immunity
Active immunity is so named because the host plays an active role in responding to the foreign antigen. The best example of active immunity is immunization, whereby a vaccine containing a foreign antigen is administered to a nonimmune host, resulting in active production of specific antibody and lymphocyte-based memory.
Passive immunity refers to transfer of soluble factors (either antibodies or cells) from an immune individual to a nonimmune host. This process confers immunity passively, without the recipient needing prior exposure to the antigen. A good example of passive immunity is parenteral administration of immune serum globulin to travelers as preexposure prophylaxis against unusual infections.
Different Parts of Immune System
Lymphocyte
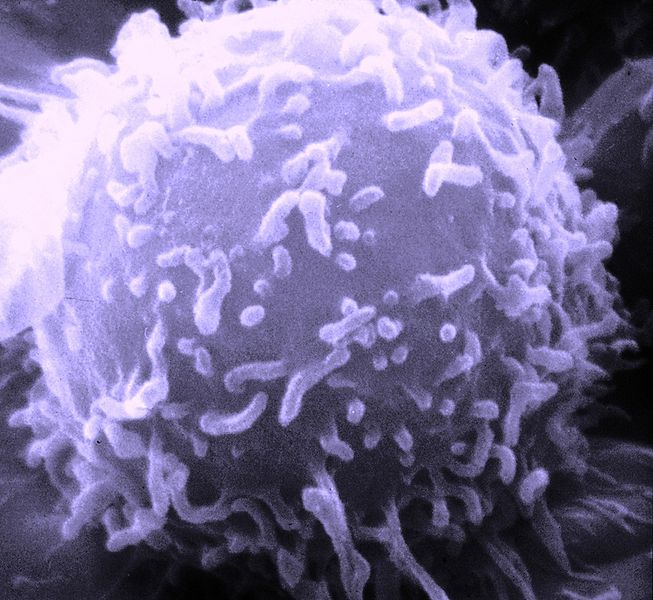
Image Credit: wikipedia.org
A lymphocyte is a type of white blood cell in the vertebrate immune system. There are two broad categories of lymphocytes, namely the large granular lymphocytes and the small lymphocytes depending on their appearance under the microscope.
Read more about Light Microscope: Parts, Usage, Handling, and Care
Read more about Electron microscope – Types, Uses, and Disadvantages
Most, but not all large granular lymphocytes are more commonly known as the natural killer cells (NK cells).
The small lymphocytes are the T cells and B cells. Lymphocytes play an important and integral role in the body’s defenses.
Natural killer cells are a part of innate immune system and play a major role in defending the host from both tumors and virally infected cells. Natural killer cells distinguish infected cells and tumors from normal and uninfected cells by recognizing alterations in levels of a surface molecule called MHC (major histocompatibility complex) class I.
Natural killer cells are activated in response to a family of cytokines called interferons. Activated natural killer cells release cytotoxic (cell-killing) granules which then destroy the altered cells.
They were named “natural killer” because of the initial notion that they do not require prior activation in order to kill cells.
Natural killer cells are not antigen-specific. They make up 5-10 percent of the lymphocyte population.
They are activated by IL-15.
On light microscopy, they have the appearance of large lymphocytes with numerous cytoplasmic granules and are sometimes called large granular lymphocytes. The granules contain substances that facilitate target cell lysis including perforin (a pore-forming protein) and granzymes. They classically express the CD16 and CD56 cell-surface markers.
Natural killer cells attack virus-infected or abnormal cells expressing low levels of class I MHC molecules, while CD8+ T cells are MHC-restricted and attack cells with a high level of class I MHC complexed to antigen.
Natural killer cells insert perforin into the cells they are attacking and secrete granzymes through the pore. Granzymes attack the cell causing apoptosis.
Natural killer cells can also kill cells through antibody-dependent cellular cytotoxicity where IgG bound to target cells interacts with Fc receptors (CD16) on NK cells causing the release of lytic enzymes. NK cells are numerous in skin biopsies of patients with graft versus host disease following bone marrow transplant. They are also increased in large granular lymphocyte (LGL) syndrome and in some patients with Felty’s syndrome.
T lymphocytes
Also known as T cells, these are Thymus-derived and express the T cell receptor on their surface. They can be separated from other lymphocytes by use of monoclonal antibodies that recognize CD3, a component of the T cell receptor. The majority of circulating lymphocytes in the bloodstream are T cells.
T cells are an important part of immunity. Depending on the roles they perform, T cells can be functionally classified into
- Helper/inducer T cells
- Suppressor T cells
- Cyto-toxic T cells
Most helper/inducer T cells express the CD4 cell-surface marker. The majority of cytotoxic T cells express the CD8 cell-surface marker.
Suppressor T cells also classically express the CD8 cell-surface marker.
The CD4+ helper T cells can be divided into two types:
Th1 Cells
These secrete IL-2 and interferon γ. These cytokines are important in cell-mediated immunity including stimulating CD8+ cytotoxic T cells to attack virally infected cells and stimulating macrophage function and granuloma formation.
Tumor necrosis factor (TNF) is also secreted promoting inflammation. IL-12 which is secreted by phagocytic cells is important for the initial induction of the Th1 response. Interferon γ inhibits Th2 cells responses.
Therapies that inhibit certain Th1 cytokines (anti TNFα) are used in Th1-mediated diseases such as rheumatoid arthritis.
Th2 Cells
These cells secrete cytokines IL-4, IL-5, IL-6, IL-10, and IL-13 which are important in humoral immunity. They perform the following functions:
- IL-4 promotes immunoglobulin class switching to IgE
- IL-5 activates eosinophils
- IL-6 promotes B cell maturation into a plasma cell.
- IL1-4, IL-10, and IL-13 inhibit macrophage activation and TNF-mediated inflammation.
α/β T cells are CD4+ T cells that express a T cell receptor with α and β chains. Most T cells have this type of receptor.
γ/δ T cells are phylogenetically older T cells comprising up to 15 percent of the T cell population in certain epithelial tissues such as skin and gut. These T cells express T cell receptors with γ and δ chains. These T cell receptors can’t recognize antigen in context with MHC.
AIDS patients have a severe dysfunction of T cell-mediated immunity and suffer from recurrent infections with these agents.
B Lymphocytes
B lymphocytes, or B cells, are bone marrow-derived antibody-secreting cells that express surface immunoglobulin on their surfaces.
B1 and B2 cells both are populations of B cells
- B1-develop earliest during ontogeny. Most express CD5. They are the source of “natural” antibodies, express high levels of IgM, are polyreactive, recognizing both common pathogens and autoantigens.
- B2-develop later in ontogeny and lack CD5 surface marker. Before encountering antigen, mature B2 cells co-express IgM and IgD antibodies on their surface. With antigen stimulation, they secrete highly specific antibody (IgM, IgG, IgA, or IgE) within the secondary lymphoid tissue.
Specific immune responses can be differentiated into two major categories based on whether B or T cells are primarily involved.
- Humoral immunity- Immune responses involving antibody that is produced by mature B cells and plasma cells.
- Cellular immunity is mediated by T cells that secrete cytokines and signal effector cells to direct an overall cell-mediated immune response.
Complement System
Complement components have immunologic activity both individually and in an activation cascade leading to a polymer formed by C5, C6, C7, C8, and C9 (the membrane attack complex, or MAC), which results in lysis of target cell membrane.
Early classic complement components (especially C3 products) act as opsonins and assist in the phagocytosis of bacterial particles by neutrophils and macrophages.
Certain complement split products (C3a and C5a) are chemotactic for phagocytic neutrophils and also act as “anaphylatoxins,” which directly stimulate mast cells and basophils to release histamine resulting in increased vascular permeability.
Deficiency of early complement components is associated with increased pyogenic infections (C3 deficiency) and an increased incidence of autoimmune diseases, possibly owing to impaired clearance of immune complexes.
The membrane attack complex appears especially important in host defense against Neisseria infection. Deficiency of any one of the terminal complement components can result in recurrent infections with Neisseria.
The complement system can be activated by three pathways:
Classical
IgM and IgG binding to antigen forming immune complexes.
Alternative
Activated by lipopolysaccharide on microbial cell surfaces in the absence of antibody. This is part of the innate immune system. IgA complexes and C3 nephritic factor can also activate this pathway.
Lectin
Lectin is secreted by the liver and binds to microbial ligands. This activates mannan-binding lectin-associated proteases.
Neutrophils and Eosinophils
Neutrophil granulocytes, generally referred to as neutrophils, are the most abundant type of white blood cells in mammals and form an essential part of the immune system. Neutrophils are important in phagocytosing and digesting foreign particles at sites of inflammation and antigen entry. Neutrophils kill and dissolve microbes by:
- Release of enzymes and bactericidal products from their intracytoplasmic granules
- By generation of toxic oxygen radicals and hypohalous acids.
Clinical deficiency of leukocytes manifests as recurrent skin and soft tissue infections with pyogenic organism and sepsis.
Eosinophils (acidophils) are white blood cells that are one of the immune system components responsible for combating infection and parasites. Eosinophils are active in immunity against parasites, especially helminths, Eosinophils are specialized leukocytes whose granules contain numerous toxic products, including major basic protein, eosinophil peroxidase, and eosinophil cationic protein.
These products are especially toxic to helminths. Activated eosinophils also produce large quantities of leukotriene C4 (LTC4) and TGF-β that promote increased venular permeability and fibroblast-dependent fibrosis, respectively.
MHC
MHC stands for major histocompatibility complex, a group of genes located on human chromosome 6. The products of MHC gene loci can be classified into two categories-class I and class II MHC molecules.
- Class I MHC molecules are expressed on the surface of all nucleated cells.
- Class II MHC molecules are found mostly on specialized cells called antigen-presenting cells.
Unlike macrophage, neutrophils, and B cells, T cells classically cannot recognize free soluble antigen. T cells can only “see” antigen via their surface T cell receptor, which will only bind to antigen bound to (“presented by”) an MHC molecule on the surface of a cell.
T cells “see” only pieces of large antigens because the antigen-binding groove in an MHC molecule can only accommodate a small peptide. Large protein antigens are digested prior to insertion in the groove.
An exception to the above involves stimulation of T cells by superantigens. A superantigen is typically a molecule of bacterial or viral origin that directly interacts with MHC class II molecules outside of the antigen-binding groove and then with the Vβ region in the T cell receptor.
The result is direct T cell activation of large amounts of T cells, all of which contain a specific Vβ region in the T cell receptor.
Therefore, the systemic host response to a superantigen can be substantial. Examples include toxic shock syndrome caused by a staphylococcal exotoxin that acts as a T cell superantigen.
Antigen Presenting Cells (APC)
Antigen-presenting cells are cells that express surface MHC (Major histocompatibility complex) class II molecules. MHC class II molecules preferentially bind to T cell receptors associated with the CD4 surface molecule. Thus, APCs present antigen to the CD4+ T cells, the helper/inducer subset.
Class I MHC molecules preferentially bind to T cell receptors associated with the CD8 surface molecule. Class I MHC molecules are present on the surface of all nucleated cells, thus allowing cells to present their internal antigens to cytotoxic T cells.
This mechanism is critically important in host defense against intracellular pathogens such as viruses.
T-cell activation requires two signals:
- Engagement of the T-cell receptor by the antigen/MHC complex
- A second signal, usually transduced by direct cognate interaction (“touching”) between costimulatory cell surface molecules on the APC and T cell.
T-cell activation resulting from the two signals leads to the production of cytoplasmic and nuclear factors, resulting in gene activation and new DNA synthesis.
Innate Immune System
The innate immune system is phylogenetically older than the acquired (specific/adaptive) immune system. Latter might take 3-5 days to be effective. Therefore, there needs to be a system capable of controlling a pathogen during that time so it doesn’t damage the host.
The innate immune system is activated immediately and can rapidly control the replication of the infecting pathogens until the lymphocytes can deal with it. Innate immune system can interact with and control the adaptive immune responses.
The strategy of the innate immune system is to recognize a few highly conserved structures present in large groups of organisms. These structures are called pathogen-associated molecular patterns (PAMP), and the receptors of the IIS that recognize them are called pattern-recognition receptors. PAMPs are produced only by microbial pathogens that may include bacterial lipopolysaccharides, peptidoglycan, and bacterial DNA/RNA.
Once the macrophages interact with PAMPs, they release cytokines (IL-1, TNFα, IL-8) that recruit and activate neutrophils and other leukocytes. Other cytokines that are released enhance microbial activities of phagocytes (interferon γ) and stimulate natural killer (IL-15) and Th1 cell mediated immune responses (IL-12, IL-18).
Pattern-recognition receptors that recognize PAMPs are mannan-binding lectin, mannose receptor, and toll-like receptors. There are at least ten mammalian toll-like receptors. They are present on cell surfaces and once stimulated, these receptors lead to activation of cell signaling pathways that results in induction of inflammatory and immune response genes with subsequent elaboration of cytokines.
Interaction with the acquired immune system
Pattern recognition receptors on antigen-presenting cells of the innate immune system bind pathogens, which are endocytosed. The phagocytized pathogen can then be processed and presented to T cells in the context of MHC class II molecules.
Furthermore, binding of PAMPs to toll-like receptors signals the APC to upregulate CD80 (B7-1)/CD86 (B7-2) costimulatory molecules on the surface of the APC. Only when the APC presents both class II molecules with antigen to the T cell receptor and CD80 (B7-1) or CD86 (B7-2) to CD28 on the T cell can the T cell be activated resulting in stimulation of the acquired immune system.
Self-antigens are not recognized by receptors of the innate immune system.
Immune Response
Immune response can be segregated into four main types
Type I – IgE-mediated immediate hypersensitivity (e.g., allergic rhinitis or hay fever)
Type II – Antibody-mediated tissue injury, (e.g., autoimmune hemolytic anemia)
Type III –Immune complex (antigen-antibody) formation (e.g., serum sickness, Arthus skin reaction)
Type IV – Delayed-type hypersensitivity (e.g., immune response to mycobacterial antigens, positive PPD skin test)
